The Boron Hydrides
Click any image to download full size.

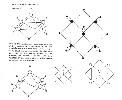



![]() Okay, not exactly nuclear data, but it is of interest to chemists, and it
might be useful in testing the shell models. I spent some time studying the
boron hydrides because of boron's semiconducting properties and its unique
electron-deficient bonds. Its the oddball stuff that leaps out at you and
promises you discoveries, if only you can figure it out, and boron is
clearly one of these oddballs. The pages above were originally
published in my 1989 report, Time and Geometry: A Geometrical Unification
of the Four Forces. At the time I did not yet have a solution for boron,
but as you can see, I was trying to gleen insights into the boron nucleus
from its bond geometry. (I never liked that shared electron junk in the
chemistry books, seemed to me like a lot of dancing around to prop up an
inadequate physical model.) I had already worked out a theory concerning the
hybridization of metallic bonds I called "linearization," and in these diagrams
I was attempting to apply the technique to the boron hydrides to see if it
would adequately explain the geometric properties of the molecules. I hoped in
the process to acquire some insights into the string geometry of boron's nuclear
structure. But, because I did not yet have the nuclear geometry of boron,
and could make no firm conclusions, and presented the data to physicists rather
than chemists, the work was pretty much ignored.
Okay, not exactly nuclear data, but it is of interest to chemists, and it
might be useful in testing the shell models. I spent some time studying the
boron hydrides because of boron's semiconducting properties and its unique
electron-deficient bonds. Its the oddball stuff that leaps out at you and
promises you discoveries, if only you can figure it out, and boron is
clearly one of these oddballs. The pages above were originally
published in my 1989 report, Time and Geometry: A Geometrical Unification
of the Four Forces. At the time I did not yet have a solution for boron,
but as you can see, I was trying to gleen insights into the boron nucleus
from its bond geometry. (I never liked that shared electron junk in the
chemistry books, seemed to me like a lot of dancing around to prop up an
inadequate physical model.) I had already worked out a theory concerning the
hybridization of metallic bonds I called "linearization," and in these diagrams
I was attempting to apply the technique to the boron hydrides to see if it
would adequately explain the geometric properties of the molecules. I hoped in
the process to acquire some insights into the string geometry of boron's nuclear
structure. But, because I did not yet have the nuclear geometry of boron,
and could make no firm conclusions, and presented the data to physicists rather
than chemists, the work was pretty much ignored.
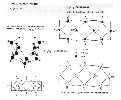
![]() The bottom line on these old diagrams is that I haven't found the
time to go back and check the shell models against them. There may be
pinning errors on them. At the time I did these I was not aware of too many
shell structures, and I thought that all covalent bonds were mediated by
radical strings and were doubly-pinned bonds. That turns out not to be the
case. Singly-pinned and un-pinned covalent bonds seem to be available also,
the doubly-pinned bond would likely be shorter and stronger than a singly-pinned
bond, and the un-pinned would be largely ionic and less rigidly spaced. I
recall that there were things that troubled me about these models. I do have
them un-folded pretty good, and the electron count and positioning are good,
but the string configurations of the covalent bonds may be incorrect. I think
the linearized bonds (daisy-chains) are correct. A couple of shell stacking
and pinning solutions for boron will tell the story, it would also be
enormously helpful if somebody could verify which isotope(s) of boron were
involved... that information was noticeably and frustratingly absent from the
chemistry texts I perused. Oh, you might also notice a modified Lewis diagram
in the images. I put the dots down to indicate electron positions. If this
theory pans out, additional enhancements to the Lewis diagrams might be
convenient, to indicate the pinning status of covalent and ionic bonds.
The bottom line on these old diagrams is that I haven't found the
time to go back and check the shell models against them. There may be
pinning errors on them. At the time I did these I was not aware of too many
shell structures, and I thought that all covalent bonds were mediated by
radical strings and were doubly-pinned bonds. That turns out not to be the
case. Singly-pinned and un-pinned covalent bonds seem to be available also,
the doubly-pinned bond would likely be shorter and stronger than a singly-pinned
bond, and the un-pinned would be largely ionic and less rigidly spaced. I
recall that there were things that troubled me about these models. I do have
them un-folded pretty good, and the electron count and positioning are good,
but the string configurations of the covalent bonds may be incorrect. I think
the linearized bonds (daisy-chains) are correct. A couple of shell stacking
and pinning solutions for boron will tell the story, it would also be
enormously helpful if somebody could verify which isotope(s) of boron were
involved... that information was noticeably and frustratingly absent from the
chemistry texts I perused. Oh, you might also notice a modified Lewis diagram
in the images. I put the dots down to indicate electron positions. If this
theory pans out, additional enhancements to the Lewis diagrams might be
convenient, to indicate the pinning status of covalent and ionic bonds.





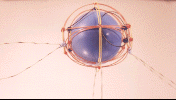
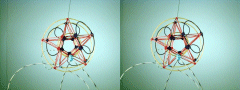
![]() Please note the hybridized orbital. Also note that the pinning of the radical
strings are preferentially placed to turn this into a
complementary shell model. The hybridized
orbital is the one pinned to the lower nuclear shell,
regardless of where you choose as its pinning site. Hybridization, in this
case, metallic linearization of the bonding string, occurs because the pinned
string is sandwiched between two quarks in adjacent complementary shells.
This flattens the lobe, essentially squeezing the lepton string in the charge
currents off the two Up quarks. (Remember that pinning of a string only emulates
a Down quark, its a way the particle can "fudge" its cosmological spreadsheet,
the quarks which have pinned strings are still essentially Up quarks at the fine
structural level.) To maintain the complementary shell structure, if you move the
hybridized orbital on the lower shell to a different up quark, you'll have to
re-locate the pinning site of the displaced lobe on the upper shell. Keep in mind
that the pinning sites are somewhat flexible, and the photo's above represent a
B10 nucleus which can pin its orbitals three different ways, but those three
results all have one linearized and two normal lobes. Inverting the shell order
as described above will yield three more pinning solutions, but in that situation
all the solutions will have two hybridized (from the lower shell) and one normal
orbital lobe (from the upper shell).
Please note the hybridized orbital. Also note that the pinning of the radical
strings are preferentially placed to turn this into a
complementary shell model. The hybridized
orbital is the one pinned to the lower nuclear shell,
regardless of where you choose as its pinning site. Hybridization, in this
case, metallic linearization of the bonding string, occurs because the pinned
string is sandwiched between two quarks in adjacent complementary shells.
This flattens the lobe, essentially squeezing the lepton string in the charge
currents off the two Up quarks. (Remember that pinning of a string only emulates
a Down quark, its a way the particle can "fudge" its cosmological spreadsheet,
the quarks which have pinned strings are still essentially Up quarks at the fine
structural level.) To maintain the complementary shell structure, if you move the
hybridized orbital on the lower shell to a different up quark, you'll have to
re-locate the pinning site of the displaced lobe on the upper shell. Keep in mind
that the pinning sites are somewhat flexible, and the photo's above represent a
B10 nucleus which can pin its orbitals three different ways, but those three
results all have one linearized and two normal lobes. Inverting the shell order
as described above will yield three more pinning solutions, but in that situation
all the solutions will have two hybridized (from the lower shell) and one normal
orbital lobe (from the upper shell).

Home
Chemistry Notes
Key to Nuclear Models
Nuclear Shells
Empirical Laws
How to Build Them
Mensuration
Glossary